What the 6th Gene Therapy for Ophthalmic Disorders Summit Was About?
With vision loss progressing rapidly in many eye diseases, and irreversible damage threatening patients with slower-onset conditions, gene therapies represented a critical one-time intervention to preserve sight.
The 6th Gene Therapy for Ophthalmic Disorders Summit brought together the latest breakthroughs driving this urgent field forward. The 2025 agenda featured exclusive preclinical data from emerging leaders such as Opus Genetics, Coave Therapeutics, and Axovia Therapeutics, alongside 2025 clinical updates from Ocugen, Adverum Biotechnologies, and Beacon Therapeutics.
Don’t miss groundbreaking data reveals and our dedicated focus on securing funding, advancing AAV delivery, and optimizing patient retention strategies - factors that are revolutionizing trial outcomes and shaping regulatory success. Register your interest for the 2026 event now!

There is a significant unmet need for innovation and curative treatments in ophthalmology. Conferences like this give passionate investors a front-row seat to where breakthroughs are happening, showcasing the progress that brings patients meaningful therapeutic solutions.
James Kasuboski, Partner and Head of Research, Luma Group, 2025 Attendee
What You Missed:
Optimizing delivery method selection through comparative analysis of suprachoroidal, subretinal, and intravitreal approaches. Learn from Abeona Therapeutics, Everads Therapy, and Ikarovec’s experiences to identify the most effective administration route for your specific gene therapy application.
Securing funding beyond clinical data requirements with investor-validated strategies. Gain insights from Luma Group on the essential company features that attract investment.
Navigating the dual challenge of endpoint development and validation through collaborative approaches. Join discussions with Beacon Therapeutics and Opus Genetics to optimize secondary endpoint collection, leverage the FDA’s RDEA program, and eliminate confounding variables for approval success.
Accessing real-time clinical trial updates from leading gene therapy developers, including Atsena Therapeutics, Adverum Biotechnologies, and 4D Molecular Therapeutics, to inform your clinical development strategies.
Mastering IND approval processes across different regulatory organizations through data-driven preclinical development approaches, and optimize model selection with insights from Coave Therapeutics, SparingVision, and Axovia Therapeutics.
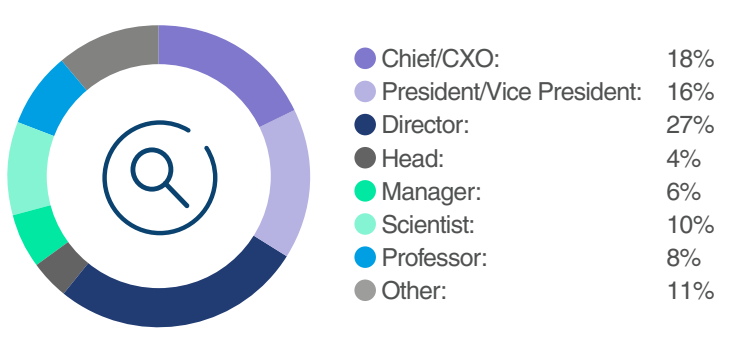
From Labs to Leadership: Who was in the Room
This forum was for expert principal scientists, directors, heads, vice presidents, and C-level executives across the ophthalmic gene therapy value chain who sought comprehensive industry insights. As the only end-to-end, industry-focused event dedicated to discovering and developing ophthalmic gene therapies, it provided unparalleled case studies and knowledge-sharing opportunities to progress this industry.