About the 5th Gene Therapy for Ophthalmic Disorders Summit
This extensive three-day event tackled obstacles spanning preclinical and clinical development. From engineering AAVs and innovating vectors for enhanced delivery and transduction to specific target cells, to confirming suitable clinical endpoints that demonstrate efficacy, all facets were covered.
Drawing in teams from R&D, preclinical, and clinical development sectors of prominent biotech and pharmaceutical companies, this gathering offered a chance to gain insights into successful strategies shared by those with first-hand experience in developing ocular gene therapies.
What Was New in 2024?
Gained insights into cutting-edge RNA-based therapeutics and gene editing therapies aimed at enhancing precision and efficacy. Learned how ASOs tackled delivery challenges with Astherna and discover how the engineered ribonucleoprotein (eRNP) CRISPR gene editor platform from Spotlight Therapeutics enabled safe and efficient gene modification.
Uncovered new data on optimizing capsids for suprachoroidal injection to address delivery hurdles with Frontera Therapeutics. Explored advancements in gene therapy vectors using the scAAVengr platform and machine learning to optimize vector performance with Avista Therapeutics.
Harnessed new technologies at the 2024 summit, including innovative synthetic AAV constructs and for non-viral gene editing showcased by Visgenx Inc., and dive into cutting-edge approaches to patient testing and clinical readiness in optogenetics for ocular disorders with Ray Therapeutics, Inc.
Discovered the latest clinical updates from Nanoscope Therapeutics, Ocugen and the BGT Foundation. Explore case studies on achieving clinical trial readiness, with insights on navigating phases 2 and 3 of trials with Adverum and Novartis ensuring safety, efficacy, and vector optimization.
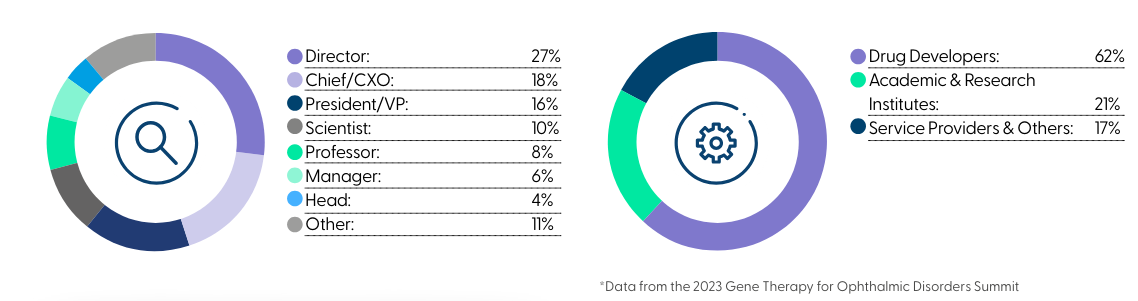
Who You Met?
Teams across R&D, preclinical and clinical development from the leading biotech and pharma companies in the space joined at the only industry-focused event tackling specific ocular gene therapy development and delivery challenges from preclinical through to clinical drug development.
Testimonials:
“Small groups and great personal interactions. It was wonderful to have the opportunity to ask and hear many questions from the community."
A Gene Therapy for Ophthalmic Disorders 2023 attendee, Kriya Therapeutics
“The conference was an exciting and impressive gathering of minds with a collective goal to push gene therapy forward. An amazing opportunity to listen in on the past and the future of the industry."
A Gene Therapy for Ophthalmic Disorders 2023 attendee, iuvo BioScience
“Diverse mix of attendees and presenters creating comprehensive coverage and fantastic expertise, small enough to facilitate audience participation, informal discussions, and excellent networking."
A Gene Therapy for Ophthalmic Disorders 2023 attendee, Duke University
