Connect with Decision Makers in Ophthalmic Gene Therapy
Partner with the only industry-led event exclusively dedicated to ophthalmic gene therapy development, uniting experts from Atsena Therapeutics, Adverum Biotechnologies, and 4D Molecular Therapeutics across the full value chain. This modality-specific conference explores the complete spectrum of gene therapy applications while remaining disease-agnostic.

Ophthalmic Gene Therapy Drug Developers Still Need Your Help With:
Rapid development of disease-specific preclinical models that accurately recapitulate ophthalmic disorder biology. Companies require expert support to overcome the time-consuming and technically challenging process of creating validated models for target validation, efficacy assessment, and toxicology testing of their gene therapies.
Comprehensive clinical trial design and management expertise. Drug developers seek specialized support for cohort recruitment, trial management, and FDA-validated endpoint development, while eliminating confounding variables that could compromise study integrity and regulatory success.
Ophthalmic-specific gene therapy delivery services ensuring precise targeting and controlled duration. With delivery errors potentially leading to inaccurate results and patient safety risks during clinical trials, developers require specialized expertise for the safe and accurate delivery of therapeutics to specific eye locations.
Why Partner in 2026?
Don’t Miss the Only Industry-Led Event Dedicated to Ophthalmic Gene Therapy Development
Showcase your preclinical models, clinical services, and delivery technologies to leading companies like Opus Genetics, Coave Therapeutics, and Ikarovec, progressing through ophthalmic gene therapy development.
Understand the latest pain points in delivery, development, and endpoints of ophthalmic gene therapies to tailor solutions for drug developers' most pressing challenges.
Network with industry-leading companies like Atsena Therapeutics, Adverum Biotechnologies, and others to build long-term relationships that accelerate ophthalmic gene therapy development.
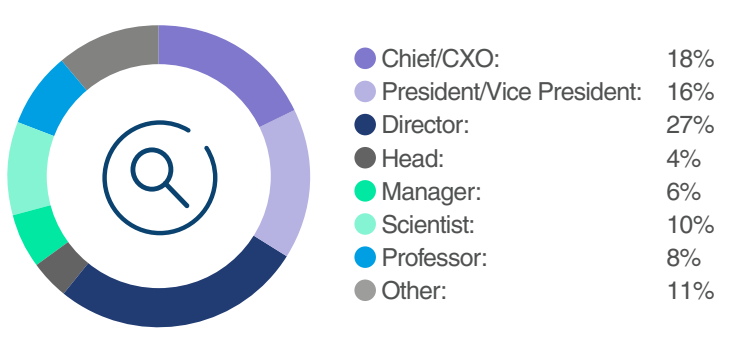
From Labs to Leadership: Who was in the Room?
You missed connecting directly with key decision-makers in preclinical and clinical development who were actively streamlining their programs to deliver the next breakthrough since Luxturna. With intensifying competition among developers and urgent time constraints to prevent irreversible vision loss, attendees were primed for solutions that accelerated therapeutic development timelines.

Explore Other Related Events
The Gene Therapy Event Series spans in-depth content for each role and specification in gene therapy drug development, so you can curate your event calendar to meet industry leaders most relevant to your area of expertise across the year. Download our Partnership Prospectus now to find out more.

